Current Research Interests
The MicroParticle PhotoPhysics Lab(MP3L) is interested in the optical frequency
interactions between molecular entities and nano-particles with micro-particles.
The interactions include external as well as internal sources. Fluorescence and
Raman scattering are of great interest as well as reactive interactions that
lead to perturbations in Whispering Gallery Modes (e.g. adsorptions of atoms,
small molecules, macro-molecules (e.g. protein, DNA) and nano-particles (e.g.virus,
quantum dots)).
Our perturbation studies are responsible for the first demonstration of a
Whispering Gallery Mode bio-sensor.
In its 27 year history the laboratory has developed a number of new
spectroscopies for studying isolated micro-particles (e.g. levitation,
evanescent guided wave interactions) and found a host of new effects. The MP3Lís
major results are detailed in
over 75 refereed publications.
The laboratory welcomes undergraduate students with proper qualification to be
mentored for senior theses by graduate students, post-doctoral fellows and
faculty. We are proud of the numerous undergraduates and masters students who
have gone onto the Ivy League, as well as others who have started hi-tech
industries, and joined major companies.
Past Research Interests

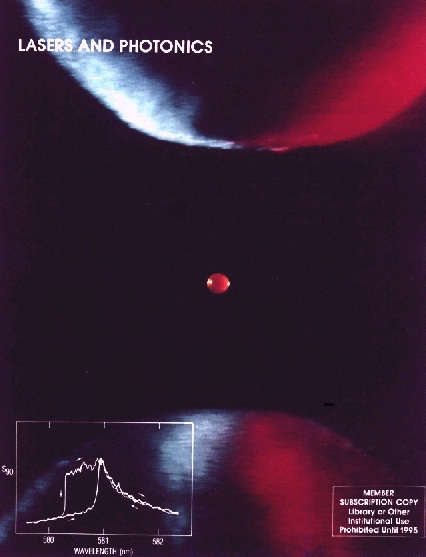
Figure 1: A fluorescent microparticle (60 micron diameter) levitated in a quadrupole meso-trap. The particle, which contains Rhodamine 6G dye, is illuminated from the side at 488 nm. The three points of light within the quasi-uniform fluorescence are caused by photographic film bleaching due to elastic scattering (i.e. glare spots). Glare spots result from combinations of reflections and refractions of laser radiation by the droplet. [Publication 28]
What is Microparticle Photophysics?
Microparticle photophysics deals with the study of laser interactions with micron and submicron sized particles. Investigations deal with photoemission, photophoretic force measurements, infrared spectroscopy, Raman spectroscopy and fluorescence spectroscopy. Current research deals with fluorescence of dye molecules within and on the surface of levitated droplets. For surfactant species, polarization selected images may be used to learn about the orientation of various fluors on the surface of an aerosol particles. Experiments in intermolecular energy transfer have looked at the way in which molecules "communicate" with each other by taking advantage of the global electromagnetic modes of the particle.
Why levitate?
Levitation of microparticles provides a neat and clean means for isolating single particles and clusters for photophysical measurements. The droplets maintain a spherical shape and isolation by levitation ensures that they are not contaminated (i.e., by contact with surfaces). Although droplets have been studied in flowing streams, levitation allows one to examine the system over an extended period of time (i.e., minutes, hours, days).
How is it done?
Microparticles are produced using an on-demand jet [Publication 19], and are inductively charged before entering the top of a Paul trap. The Paul trap consists of two electrodes which are hyperboloids of revolution and a torus with a hyperbolic cross-section. The bottom and top electrods allow for balancing the droplet against gravitational forces with an electrostatic field, while an oscillating potential applied to the torus facilitates trapping. The oscillating quadrupole field which results, confines the particle to the center of the levitator-trap through alternating gradient forces.
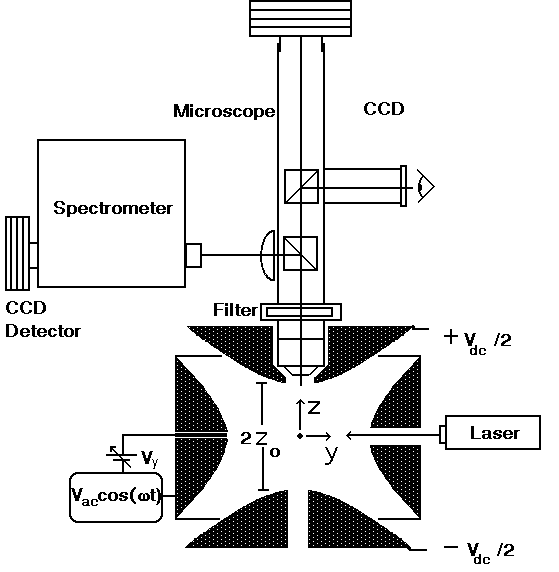
Figure 2: The Aerosol Particle Microscope- Spectrometer. The Paul trap is used for containing the microparticles in space so that they may be investigated through imaging and spectroscopy.
What's that noise?
Since much of the work done in the Microparticle Photophysics Laboratory is performed in air, the levitated droplets are subject to collisions with molecules in the air. When the particle become sufficiently small, these collisions are readily observe d in the random motion of the particle around the center of the trap. These Brownian forces knock the particle from the stable center leading to noise in microphotographs of the droplet. A technique known as Squeezed Imaging has been developed [Publication 49] which allows one to take advantage of the phase dependence of the variance to improve the quality of long-term images down to the optical resolution of the microscope.
Imaging as a means for measurement?
From the moment we first opened our eyes, we began imaging. Certainly, the fastest method to learn about something is simply to look at it. At the Microparticle Photophysics Laboratory, we have found that one can make investigations of "in situ" aerosol particles through electronic imaging. Experimental investigations into elastic scattering, absorption spectroscopy [Publication 46], intermolecular energy transfer [Publication 52] and orientation of surfactant species [Publication 51] have been performed using imaging on a CCD. These investigations have been per formed on particles that were not significantly affected by the Brownian forces, and thus the images obtained were not compromised. With the introduction of the Squeezed Imaging technique, it is now possible to perform the same experiments on Brownian particles while maintaining image quality.
Microparticle studies at the Polytechnic
Work at the Microparticle Photophysics Laboratory (MP3L) is involved in the study of laser interactions with micron and submicron sized particles while levitated in Quadrupole traps or on surfaces. The laboratory has grown into three separat e disciplines. In the traditional MP3L work on the interation of excited states with macroscopic structures is the major focus. Here we have discovered enhanced intermolecular energy transfer by a QED mechanism. In the Microparticle Photonics Lab in Electrical Engineering the interest is in the interaction between guided waves and microparticles. Finally in the Microparticle Nucleation Lab in Chemical Engineering the interest is in the embryos which are responsible for crystal growth in supersaturated solutions. Work is sponsored in these three laboratories by the NSF, NASA and the AirForce.
Recent Patent (5,532,140)
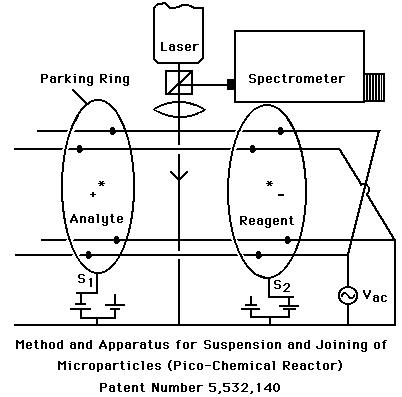
Figure 3: The pico-chemical reactor is a recent patent that provides a means for suspending and joining microparticles for chemical analysis.
The pico- chemical reactor is the first device for joining an unknown pico-liter droplet with a reagent for chemical analysis through optical spectroscopy. The device levitates particles of opposite charge by using alternating gradient forces. Electrostatic rings "park" both the analyte droplet (shown on left) and the reagent droplet (shown on right). By stepping potentials of the rings to the opposite state, the particles are forced together from their parked positions. The resulting collective droplet represents the equivalent of mixing materials within a testube, however, the vessel is isolated and only ~10 millionths of a meter in size (~1 picoliter). Analysis is anticipated through laser spectroscopy of inelastic light scattering (e.g., Raman or Fluorescence).